Gene therapy for hemophilia represents a groundbreaking advancement in the treatment of this challenging condition. With the recent FDA approval of Hemgenix therapy, patients like Terence Blue are finally able to experience a world where constant worry over bleeding episodes and daily injections may become a thing of the past. The benefits of gene therapy for hemophilia extend beyond merely reducing the need for clotting factor; they promise to transform the lives of those living with hemophilia by addressing the root causes of their condition. As medical technology advances, the long-awaited dream of effective hemophilia treatment is becoming a reality for many. This novel approach offers hope for not just managing symptoms, but potentially providing lasting solutions for patients affected by this disorder.
The evolution of treating hemophilia is now embracing innovative methods, particularly through genetic interventions. Gene therapy, a term that includes modules like Hemgenix, aims to provide a solution that addresses the deficiencies at the genetic level, thus revolutionizing how we approach hemophilia care. Patients, many of whom have spent years managing their condition with regular infusions, are finding renewed hope in these advanced therapies. This shift signifies a movement towards more permanent solutions for bleeding disorders, bringing with it increased possibilities for a healthier lifestyle. As we continue to explore these new frontiers in hemophilia treatment, it’s essential to embrace the potential they hold for those living with this historically daunting condition.
Understanding Hemophilia and Its Challenges
Hemophilia is a genetic bleeding disorder that affects the body’s ability to clot blood is crucial for preventing excessive bleeding. This condition is predominantly inherited and is characterized by deficiencies in clotting factors in blood. While hemophilia can vary in severity, most people affected by it face constant management challenges, including the need for regular infusions of clotting factors. The emotional toll is significant, as those living with hemophilia might experience anxiety over potential injuries, and the fear of bleeding episodes can often overshadow daily activities. Awareness and education about hemophilia treatment options are essential for enhancing the quality of life for those living with this condition.
Living with hemophilia means adopting a proactive approach to health and safety. Patients like Terence Blue navigate life with caution, carefully assessing risks in various activities. Advances in treatment options, such as prophylactic factor infusions, have significantly improved the prognosis for many, allowing individuals to pursue more active lives. Nevertheless, every bleeding episode can still pose a serious health risk, which reinforces the importance of maintaining routine care and staying informed about new and emerging treatments for hemophilia.
Gene Therapy for Hemophilia: A New Frontier
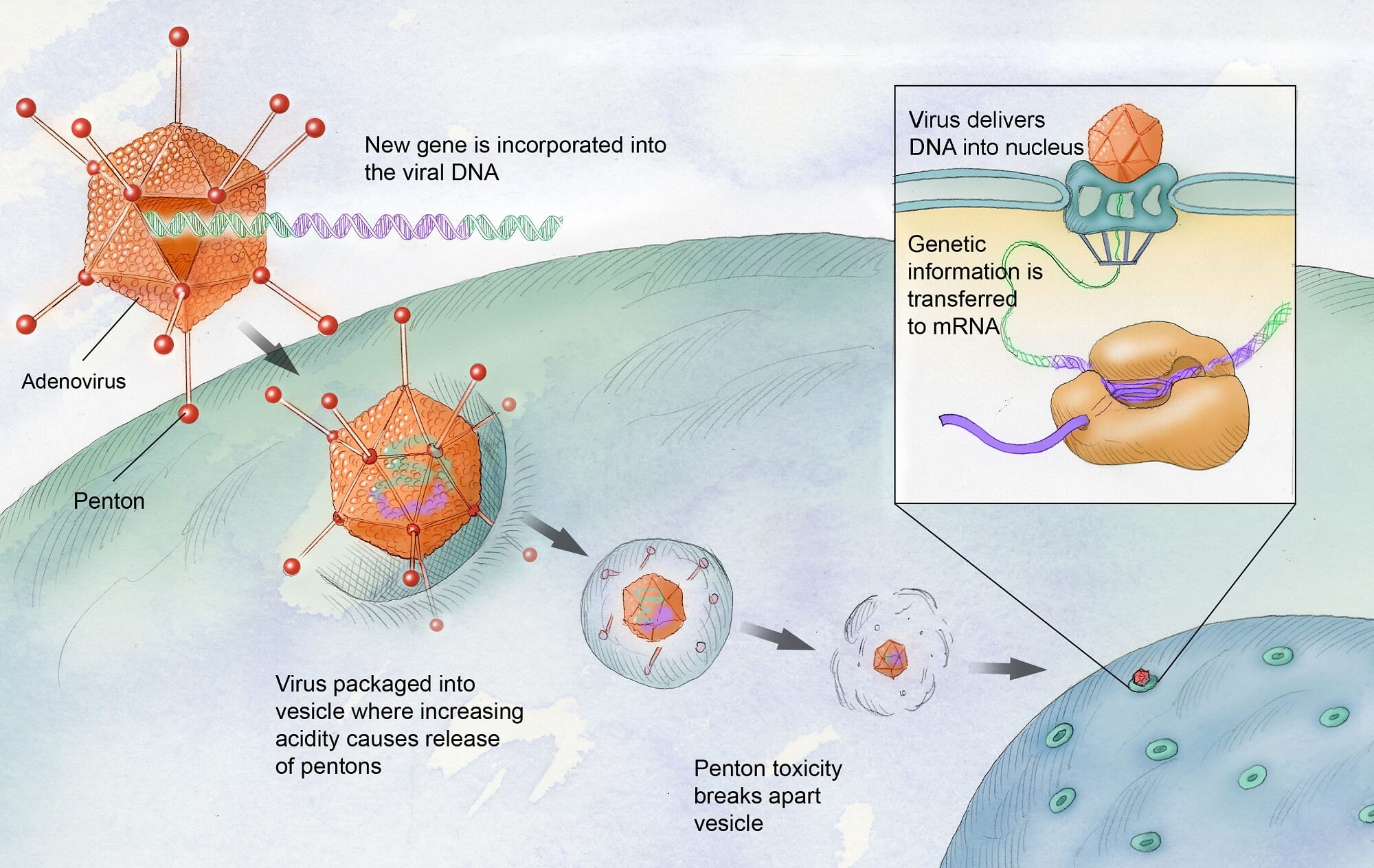
Gene therapy has emerged as a groundbreaking option for those suffering from hemophilia, particularly with the introduction of treatments like Hemgenix. This innovative therapy aims to correct the underlying genetic defect responsible for hemophilia B, potentially providing lasting benefits without the need for continuous infusions of clotting factors. By using engineered viruses to deliver therapeutic genes directly into the liver, Hemgenix offers a promising alternative to traditional therapies and has gained FDA approval, paving the way for future advancements in gene therapy for hemophilia and beyond.
The benefits of gene therapy extend beyond merely alleviating the burden of regular treatments. For patients like Terence Blue, it represents the possibility of a more liberated lifestyle without the constant fear of bleeding or the need for emergency supplies. Gene therapy not only addresses the immediate symptoms of hemophilia but also offers hope for a long-term solution that could fundamentally change how patients live with this condition. Continued research and innovation in this field suggest that more patients may gain access to potentially curative therapies, marking a significant milestone in hemophilia treatment.
The Impact of FDA Approval on Hemophilia Treatments
The FDA approval of therapies such as Hemgenix is a landmark achievement in the realm of hemophilia treatment. It signifies not only the culmination of rigorous clinical trials but also the potential for transformative change in how hemophilia is managed. With the assurance of safety and efficacy provided by the FDA, more healthcare providers are likely to adopt gene therapies as viable treatment options, thereby expanding access for patients who have long relied on traditional methods. As patients and clinicians embrace these new options, it is crucial to remain informed about their implications and integration into standard care practices.
However, the journey to widespread acceptance of gene therapy comes with challenges. Pricing remains a contentious issue, as the high costs associated with these therapies can limit availability to many patients. The healthcare system must navigate the balance between innovation, efficacy, and affordability to ensure equitable access to groundbreaking treatments. Advocacy for patient perspectives and experiences is vital in addressing these inequities and promoting policies that support sustainable practices in the deployment of new hemophilia therapies.
Living with Hemophilia: Beyond Treatment
For many individuals diagnosed with hemophilia, life extends beyond regular treatments and medical appointments. The social dynamics of living with a bleeding disorder can be complex, as concerns about injury and bleeding can influence personal relationships and lifestyle choices. Many individuals with hemophilia, like Terence Blue, must navigate the intricacies of explaining their condition to friends, colleagues, and family, often facing misunderstandings and stigma along the way. Support networks and community programs play an essential role in helping patients manage the social aspects of hemophilia, fostering understanding and solidarity among those affected.
Furthermore, living with hemophilia requires personal resilience and adaptability. Patients must develop strategies for managing their health while still engaging in activities they enjoy. For instance, many find ways to participate in sports or physical recreation safely by taking precautions and educating themselves on injury prevention. By advancing personal education and continually advocating for awareness, individuals with hemophilia can lead fulfilling lives while managing their condition, demonstrating that the journey involves more than just medical treatment.
The Future of Hemophilia Treatments
Looking ahead, the landscape of hemophilia treatment is poised for significant transformation, driven largely by advancements in gene therapy and cell-based treatments. These innovative approaches hold great promise for the next generation of therapies that may drastically reduce the need for frequent intervention and improve the quality of life for patients. As research continues to unveil new findings and refine existing treatments, the prospect of finding more targeted, effective solutions for hemophilia becomes increasingly feasible.
Moreover, collaboration between researchers, pharmaceutical companies, and advocacy groups is crucial in shaping the future of hemophilia care. This synergy will ensure that breakthroughs turn into practical treatments available to patients who need them most. As gene therapy technologies evolve, patients and their families can remain hopeful for a future where living with hemophilia does not equate to enduring constant injections and limitations, allowing for a more liberated and normal lifestyle.
Patient Experiences with Gene Therapy
The stories of individuals like Terence Blue illustrate the profound impact of gene therapy on daily life for hemophilia patients. As the first person in New England to receive the Hemgenix treatment, Blue’s journey highlights the hope that gene therapy brings to countless others in similar situations. His experience encapsulates both the excitement of potential healing and the palpable anxiety that comes with trying something so new. For him, the ability to live life with fewer concerns and limitations represents a tangible shift towards enhanced well-being.
Further, patient experiences are crucial in assessing the real-world implications of gene therapy for hemophilia. They offer insights into how these treatments affect everyday life — from physical activities to social interactions. Successful outcomes can inspire others with hemophilia to consider these new options and seek the care they need without the weight of traditional treatment barriers. Sharing these stories within communities can foster greater understanding and acceptance of gene therapies, leading to widespread advocacy and improved healthcare policies.
Challenges Facing Gene Therapy Adoption
Despite the optimistic outlook for gene therapy for hemophilia, several challenges remain in the widespread adoption and integration of these treatments. Among these challenges are the prohibitive costs and the complexity of gene therapy mechanisms, which can create hesitance among both patients and healthcare providers. Additionally, misunderstandings about the nature of gene therapy can lead to apprehension about safety and long-term effectiveness, posing barriers to treatment access despite FDA approvals.
Moreover, the healthcare system must grapple with balancing innovation with the economic realities of treatment options. As gene therapies like Hemgenix offer transformative potential, addressing the sustainability of prices and ensuring equitable access for all patients remains paramount. Continuous education efforts for physicians and patients alike are essential — activating informed discussions can help demystify gene therapies and promote acceptance and understanding, ultimately impacting treatment preferences in the hemophilia community.
The Importance of Ongoing Research
As we move forward in the exploration of gene therapy for hemophilia, ongoing research and clinical trials are essential in refining these treatments and addressing any emergent challenges. The development of more effective gene therapies hinges on better understanding the genetic underpinnings of hemophilia and how best to deliver gene therapies into the patient’s body. Investment in research is crucial in ensuring new solutions are tailored to meet diverse patient needs, offering hope for future breakthroughs.
Moreover, supporting and expanding clinical trials allows researchers to gather valuable data and insights necessary for improving existing treatments and developing innovations that address the unmet needs of hemophilia patients. Collaborative efforts between universities, research institutions, and healthcare providers will be paramount in accelerating advancements and fostering an environment where new therapies can be translated efficiently from laboratory to clinical practice.
Navigating Insurance and Accessibility in Gene Therapy
Navigating the insurance landscape is critical for hemophilia patients looking to access gene therapy. With treatments like Hemgenix carrying high price tags, understanding how insurance coverage works is vital for patient access and affordability. Many healthcare providers are working closely with patients to help them navigate insurance policies and explore options that could mitigate out-of-pocket costs, as coverage can vary greatly depending on the provider and the individual’s plan.
Moreover, advocacy plays a fundamental role in pushing for policies that enhance access to gene therapies for hemophilia patients. Organizations and support groups focused on hemophilia can provide resources, educate patients about their rights, and champion changes in healthcare policies that will ensure meaningful access to essential treatments. As gene therapy becomes a more significant part of the hemophilia treatment landscape, unified action will be essential in lifting the barriers that many patients currently face.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
Gene therapy for hemophilia, specifically Hemgenix, is a cutting-edge treatment that aims to correct the genetic mutation responsible for hemophilia B. It utilizes a virus to deliver a normal copy of the clotting factor IX gene directly into the liver cells, where it prompts the production of the missing clotting factor. This approach can potentially reduce or eliminate the need for regular clotting factor infusions, significantly improving the quality of life for patients living with hemophilia.
What are the benefits of gene therapy for hemophilia compared to traditional treatments?
Gene therapy for hemophilia, like Hemgenix, offers several benefits over traditional treatments. Unlike regular clotting factor injections, which are typically required several times a week, gene therapy is administered as a single treatment designed to provide long-term effects. Patients could experience a marked decrease in bleeding episodes and an improved overall quality of life, as seen in clinical trials where most participants did not require prophylactic treatments after receiving the therapy.
Is Hemgenix therapy FDA approved for hemophilia treatment?
Yes, Hemgenix therapy has received FDA approval for the treatment of hemophilia B. Approved in November 2022, it represents a significant advancement in hemophilia treatment by offering a one-time gene therapy option aimed at addressing the underlying cause of the disorder, rather than just managing its symptoms.
What should patients expect during the gene therapy process for hemophilia?
Patients receiving gene therapy for hemophilia, such as Hemgenix, can expect an outpatient procedure where the therapy is infused into the bloodstream. This process typically takes around two hours, followed by a brief observation period. Post-treatment, patients might experience some side effects, but many report minimal issues. Continuous monitoring of factor IX levels will be conducted to assess the therapy’s effectiveness.
How does living with hemophilia change after undergoing gene therapy?
After undergoing gene therapy for hemophilia, many patients experience a significant change in their daily lives. For instance, the need for regular clotting factor infusions reduces, leading to less anxiety around bleeding incidents and a more active lifestyle. Patients report feeling liberated from the constant management that hemophilia demands, enabling them to engage in more physical activities and socialize without the worry associated with their condition.
What are the financial considerations for gene therapy treatments like Hemgenix?
Gene therapy treatments, including Hemgenix, are associated with substantial costs, with Hemgenix priced around $3.5 million. Discussions with healthcare providers about financial assistance options, insurance coverage, and potential negotiation of prices with payers can provide clarity for patients. It’s essential for patients to understand these financial implications before proceeding with treatment.
What are the long-term effects of gene therapy for hemophilia?
The long-term effects of gene therapy for hemophilia look promising, with data showing that a high percentage of treated individuals maintain normal levels of factor IX years after receiving therapy. While it is not labeled a ‘cure’ as individual responses may vary, the treatment has led to sustained improvement in clotting factor levels, indicating potentially lasting benefits for patients.
How do patients feel about the potential of gene therapy for hemophilia?
Patients often express a sense of hope and relief regarding the potential of gene therapy for hemophilia. Many view it as a significant breakthrough that could change their long-term management of the condition. Reports from trial participants indicate a decrease in their previous limitations imposed by hemophilia, fostering a newfound optimism about living a more unrestricted and healthy life.
| Key Point | Details |
|---|---|
| Terence Blue’s Experience | First patient in New England to receive gene therapy for hemophilia B. |
| Gene Therapy Overview | Hemgenix, approved in November 2022, focuses on treating hemophilia B by introducing corrected genes into the liver. |
| Benefits of Therapy | Potential for long-term reduction in bleeding episodes and less dependence on regular clotting factor injections. |
| Market Challenges | High costs and market pressures may limit patient access and subsequent availability of gene therapies. |
| Regulatory Landscape | FDA approved multiple therapies, marking rapid developments in gene therapy for blood disorders. |
Summary
Gene therapy for hemophilia is revolutionizing patient care, as illustrated by Terence Blue’s transformational experience with Hemgenix. This innovative treatment not only provides hope for reducing the dependency on chronic factor injections but also promises healing capabilities that were once viewed as a distant dream. With continuing advancements in gene and cell therapies, the potential to significantly alter the lives of those affected by hemophilia has never been more tangible.