TIM-3 therapy for Alzheimer’s is an innovative approach that leverages insights from cancer immune therapy to combat one of the most devastating neurodegenerative diseases. Recent research has shown that targeting the TIM-3 checkpoint molecule can rejuvenate brain immune cells known as microglia, enhancing their ability to clear amyloid plaques that contribute to cognitive decline. This breakthrough offers new hope for Alzheimer’s treatment by potentially restoring memory functions that are typically impaired in affected individuals. With TIM-3 therapy, the anti-TIM-3 antibody could unlock the fight against Alzheimer’s by reactivating the brain’s immune response, which, when dysfunctional, plays a critical role in plaque accumulation. Given the rising prevalence of Alzheimer’s, this novel strategy underscores the need for innovative therapies that could effectively tackle the disease at its root.
The application of TIM-3 therapy for Alzheimer’s disease represents a promising frontier in neurodegenerative research, intertwining elements from oncology and immunology. By strategically inhibiting TIM-3, a molecular checkpoint that regulates the immune response, scientists aim to mobilize microglia—the brain’s resident immune cells—to eliminate harmful plaques. This method not only seeks to advance Alzheimer’s treatment but also explores the intricate dynamics between brain immune cells and neurodegenerative processes. As researchers delve deeper into the implications of TIM-3 modulation, the potential to develop effective therapies that can restore cognitive functions and alter the trajectory of Alzheimer’s disease becomes increasingly tangible. This research paves the way for improved patient outcomes and offers a fresh perspective on addressing the complexities of brain health.
TIM-3 Therapy for Alzheimer’s: A New Hope
Research indicates that TIM-3 therapy may hold promise for treating Alzheimer’s disease, especially in cases of late-onset AD, which accounts for 90-95% of all instances. By targeting the TIM-3 molecule, scientists can unlock the potential of microglia, the brain’s primary immune cells, which are often rendered ineffective due to high levels of TIM-3 expression in Alzheimer’s patients. This connection is critical because effective plaque clearance by microglia could significantly improve cognitive functions and memory, which are typically impaired in Alzheimer’s.
The therapy would ideally involve using an anti-TIM-3 antibody or a small molecule that inhibits TIM-3 activity, thus allowing microglia to regain their function of clearing amyloid plaques from the brain. Initial studies in genetically modified mice have shown remarkable improvements in cognitive abilities following the deletion of TIM-3, which suggests that similar approaches could be tested in humans. Harnessing the body’s immune response to manage Alzheimer’s symptoms presents a novel strategy that diverges from traditional treatments.
Understanding the Role of Microglia in Alzheimer’s
Microglia are often referred to as the brain’s immune cells, performing vital roles in maintaining brain health and function. In the context of Alzheimer’s disease, the presence of amyloid plaques leads to microglia becoming hyperactive, with elevated expression of the checkpoint molecule TIM-3. This inhibits their ability to clear plaques, exacerbating the disease’s progression. Understanding the mechanisms by which microglia become dysfunctional is crucial for developing therapies that can restore their normal pruning and clearing functions.
Typically, microglia are responsible for synaptic pruning during development, helping to maintain healthy brain function by eliminating useless synapses. However, in older adults, these cells’ functionality declines. They become homeostatic and cease effective clearance operations when stimulated by high TIM-3 levels. This not only affects memory retention but also contributes to the overall pathology associated with Alzheimer’s, making the exploration of TIM-3 inhibition a pivotal focus of contemporary Alzheimer’s research.
The Mechanism of Checkpoint Molecules and Alzheimer’s Disease
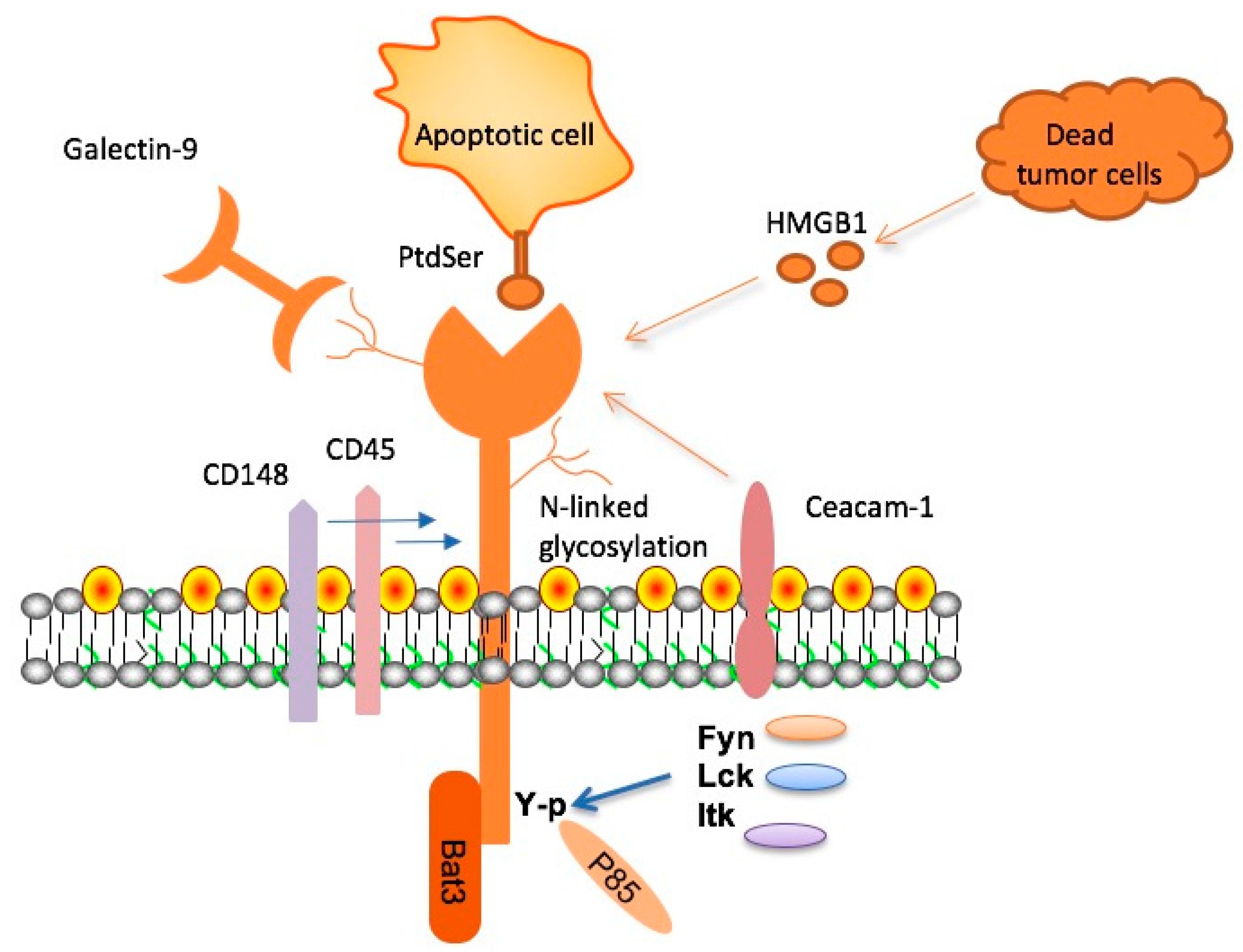
In the immune system, checkpoint molecules like TIM-3 are essential for regulating immune responses, preventing over-activation that could lead to autoimmunity. In the case of Alzheimer’s, however, the suppression induced by TIM-3 prevents microglia from responding adequately to accumulated plaques in the brain. This presents a paradox where a normal immune response becomes detrimental to brain health. Thus, understanding how TIM-3 operates within the context of Alzheimer’s can provide insights into a viable intervention strategy.
The concept of using checkpoint inhibitors, traditionally utilized in cancer therapy to unleash T-cell responses against tumors, is now being adapted for Alzheimer’s treatment. This innovative approach leverages the body’s existing immune mechanisms to combat the neurodegenerative processes at play. By alleviating the inhibitory influence of TIM-3 on microglia, researchers hope to not only enhance plaque clearance but also to restore cognitive function in affected individuals.
Emerging Research Directions on TIM-3 and Alzheimer’s
Ongoing studies are exploring the underlying genetics of TIM-3 and its association with late-onset Alzheimer’s disease. Specifically, the HAVCR2 gene polymorphism is of interest, as the variations in this gene are linked to an increased risk of developing Alzheimer’s, suggesting a clear genetic component that affects microglial function. Identifying how different gene expressions influence TIM-3 levels could pave the way for personalized approaches in Alzheimer’s treatment.
In collaboration with research institutions, scientists are delving into how anti-TIM-3 antibodies could be applied in clinical settings. Current research is investigating the efficacy of these antibodies in Alzheimer’s mouse models infused with human TIM-3 to mirror the disease environment in humans. This model provides a suitable platform for testing therapies aimed at reactivating microglial function and potentially yielding significant advancements in Alzheimer’s treatment.
The Clinical Potential of TIM-3 Inhibition
With the knowledge that TIM-3 inhibits the innate immune response in the brain, the clinical potential for TIM-3 inhibitors emerges robustly. Utilizing anti-TIM-3 antibodies not only restores the functionality of microglia but also addresses broader implications for Alzheimer’s disease management, especially given the limitations associated with current amyloid-targeting therapies. Focusing on TIM-3 will provide an avenue to explore solutions that go beyond simply targeting plaques.
Additionally, as research progresses, the advantages of TIM-3 inhibition may extend beyond Alzheimer’s to other neurodegenerative conditions characterized by immune dysregulation. Consequently, the exploration of TIM-3 as a therapeutic target holds promise for a range of cognitive disorders, thereby enhancing our understanding of the immune system’s role in neurodegeneration, offering hope to patients who currently have limited options.
Challenges in Developing TIM-3 Therapy for Alzheimer’s
Despite the promising results shown in preclinical studies, several challenges remain in translating TIM-3 therapy from bench to bedside. The safe and effective delivery of anti-TIM-3 antibodies to the brain poses a significant hurdle, as traditional drug delivery methods often fail to penetrate the blood-brain barrier adequately. Research is ongoing to identify innovative delivery systems that can ensure therapeutic levels of these antibodies reach the target site.
Moreover, the potential for immune-related adverse effects needs to be thoroughly investigated in clinical trials, as dampening immune mechanism might inadvertently lead to complications such as heightened susceptibility to infections or autoimmune responses. Balancing the therapeutic benefits of TIM-3 inhibition against these risks will be crucial in developing a robust treatment regimen.
Impacts of TIM-3 Studies on Alzheimer’s Treatment Landscape
The emergence of research on TIM-3 and its effects on Alzheimer’s disease signifies a paradigm shift in how we approach treatment strategies. With the historical challenges faced by amyloid-targeting therapies, TIM-3 presents a refreshing avenue that prioritizes the brain’s immune health. This approach to targeting innate immune dysfunction indicates a potential to fundamentally reshape patient outcomes in Alzheimer’s disease.
By broadening the focus beyond amyloid plaques to include the role of immune cells like microglia, researchers can unlock new therapeutic avenues that may lead to more substantial improvements in cognitive function and quality of life for Alzheimer’s patients. This evolving treatment landscape highlights the importance of continued research into immune pathways, ensuring that patients receive the most advanced and promising therapies in the future.
The Future of TIM-3 Research in Alzheimer’s
As research tasks advance into more intricate studies of TIM-3’s role within the Alzheimer’s disease context, interdisciplinary approaches are becoming increasingly relevant. Collaborations between neurobiologists, immunologists, and pharmacologists will be essential in refining treatment protocols that leverage TIM-3 inhibition effectively. The success of future therapies will depend on our ability to understand the complex interplay between immune response and neurodegeneration.
It is crucial to continue exploring innovative methods for assessing TIM-3 expressions and genetic influences that may alter patient outcomes in Alzheimer’s. This emerging field of study promises not only to enhance our understanding of Alzheimer’s pathophysiology but also to identify multidimensional treatments that address the underlying causes of cognitive decline. Ultimately, TIM-3 research harbors substantial potential in redefining therapeutic paradigms in Alzheimer’s care.
Interdisciplinary Collaboration: A Pathway to Innovation
The complexities surrounding TIM-3 and its implications for Alzheimer’s disease highlight the necessity for interdisciplinary collaboration. Brigid with cutting-edge research from varied expertise areas, scientists can develop innovative solutions tailored to the multifaceted nature of neurodegeneration. Such partnerships are crucial to merging insights from immunology, genetic research, and therapeutic development, thereby enhancing the overall approach to Alzheimer’s treatment strategies.
Collaboration not only accelerates research but also facilitates the sharing of resources, knowledge, and methodologies that can lead to groundbreaking discoveries. Fostering a community of researchers working harnessing TIM-3 and its potential offers hope for transformative treatments that could engage patients previously limited by ineffective standard therapies. Engaging multiple perspectives in this collaborative manner is paramount to paving the future in Alzheimer’s research.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease?
TIM-3 therapy for Alzheimer’s disease involves the use of an anti-TIM-3 antibody or small molecules to inhibit the activity of the TIM-3 molecule in microglia, the brain’s immune cells, enhancing their ability to clear amyloid plaques. This approach is based on findings that deleting TIM-3 improves cognitive function in animal models by allowing microglia to effectively remove plaque buildups associated with Alzheimer’s.
How does TIM-3 affect microglia in Alzheimer’s treatment?
In Alzheimer’s treatment, TIM-3 acts as a checkpoint molecule that inhibits microglia from attacking and clearing amyloid plaques. This inhibition leads to plaque accumulation, worsening Alzheimer’s symptoms. By targeting TIM-3 through specific therapies, researchers aim to reactivate microglia’s ability to engage and clear these harmful plaques, potentially restoring cognitive function.
What is the role of microglia in Alzheimer’s disease and TIM-3 therapy?
Microglia are the primary immune cells in the brain that protect against disease and clear debris, including amyloid plaques in Alzheimer’s. TIM-3 therapy aims to block the inhibitory effects of TIM-3 on microglia, enabling them to resume their role in clearing plaques and improving cognitive symptoms of Alzheimer’s disease.
Are there any studies supporting TIM-3 therapy for Alzheimer’s?
Yes, studies have demonstrated that deletion of the TIM-3 gene in mice models of Alzheimer’s improves cognitive function and decreases plaque burden. These findings suggest that TIM-3 therapy could be a viable option for enhancing Alzheimer’s treatment through the reactivation of plaque-clearing microglia.
What are the potential benefits of anti-TIM-3 antibodies in Alzheimer’s treatment?
Anti-TIM-3 antibodies have the potential to enhance microglia activity in Alzheimer’s disease by blocking the TIM-3 signal that inhibits plaque removal. This therapeutic strategy may lead to improved cognitive outcomes in Alzheimer’s patients, as it allows the brain’s immune cells to effectively target and clear accumulated amyloid plaques.
How does TIM-3 therapy compare to traditional Alzheimer’s treatments?
Unlike traditional Alzheimer’s treatments that focus on amyloid plaque targeting, TIM-3 therapy offers a novel approach by enhancing the brain’s immune response. It aims to tackle the root cause of plaque accumulation by empowering microglia, potentially leading to greater cognitive improvements compared to standard therapies.
What challenges might TIM-3 therapy face in Alzheimer’s disease?
TIM-3 therapy may face challenges such as ensuring the selective delivery of anti-TIM-3 antibodies to the brain and overcoming potential side effects associated with modifying immune responses. Additionally, further research is required to determine its safety and efficacy in human patients.
What are the potential side effects of TIM-3 therapy for Alzheimer’s?
Potential side effects of TIM-3 therapy may include unintended immune responses, as altering TIM-3 function could affect overall immune regulation. Ongoing research seeks to assess these risks while optimizing the therapeutic effects of reactivating microglia in Alzheimer’s.
How long has research into TIM-3 therapy for Alzheimer’s been ongoing?
Research into TIM-3 therapy for Alzheimer’s disease has been conducted over several years, with significant findings emerging from studies that aim to understand and manipulate the TIM-3 signaling pathway to benefit cognitive function in Alzheimer’s models.
What is the next step in TIM-3 therapy research for Alzheimer’s?
The next step involves testing candidate anti-TIM-3 antibodies in mouse models that replicate human Alzheimer’s pathology, to evaluate their effectiveness in inhibiting plaque development and restoring cognitive function before progressing to human clinical trials.
| Key Point | Description |
|---|---|
| TIM-3 Molecule | TIM-3 is an immune checkpoint molecule that inhibits microglial cells from clearing amyloid plaques in Alzheimer’s. |
| Role of Microglia | Microglia are brain immune cells that prune synapses and clear debris; they become dysfunctional with increased TIM-3. |
| Alzheimer’s-Related Tim-3 Polymorphism | A specific polymorphism in the TIM-3 gene (HAVCR2) increases its expression in Alzheimer’s patients, correlating with late-onset Alzheimer’s. |
| Studied in Mice | Research was conducted with genetically modified mice lacking TIM-3, which showed improved plaque clearance and memory. |
| Therapy Potential | Proposed therapy includes anti-TIM-3 antibodies or small molecules that inhibit TIM-3 to restore microglial function. |
| Future Research | Next steps involve testing human anti-TIM-3 in Alzheimer’s mouse models to evaluate its effectiveness. |
Summary
TIM-3 therapy for Alzheimer’s presents a groundbreaking approach to treatment by leveraging the immune system’s mechanisms. Research suggests that modifying TIM-3 expression can enhance microglial function, allowing them to clear harmful amyloid plaques that contribute to cognitive decline in Alzheimer’s patients. With promising results from animal studies indicating improved memory and cognitive function after TIM-3 inhibition, this therapy could pave the way for innovative treatments in humans. Continued exploration of TIM-3’s role and its translational potential may significantly influence future Alzheimer’s interventions.